CM-NANO: A single process solution to formulate poorly soluble drugs as soluble and directly compressible drug nanoparticles, is a technology to formulate poorly soluble drugs as soluble and directly compressible drug nanoparticles developed by SSPC investigator Dr Luis Padrela at the University of Limerick.
The Challenge
Poor solubility and bioavailability of new chemical entities is a major challenge for the pharmaceutical industry and jeopardizes their path to market. Approximately 75% of new pharmaceutical drugs which are worth more than $65 billion are wasted every year, as these do not reach the market due to poor bioavailability and to a lack of efficient manufacturing and formulation approaches which are capable of producing APIs with appropriate bioavailability. There is a need for efficient manufacturing and formulation approaches, which are capable of producing APIs with appropriate bioavailability.
Nanotechnologies hold a great promise to overcome these chemical barriers; however, existing methods face many technical difficulties including lack of control on the solid state and particle size of the products, poor collection yields and downstream processability of the nanoparticles.
The Solution
At SSPC, the team have developed novel batch/semi-continuous and continuous nano-spray drying/coating technologies which offer a solution to poorly soluble drugs while also addressing poor bioavailability, low efficacy and poor flowability/manufacturability. This technologies, through a single process, allows for the control of nanoparticle size, the collection of the spray-dried nanoparticles with high yields, and the conversion of these nanoparticles into micron-sized solid nanodispersions.
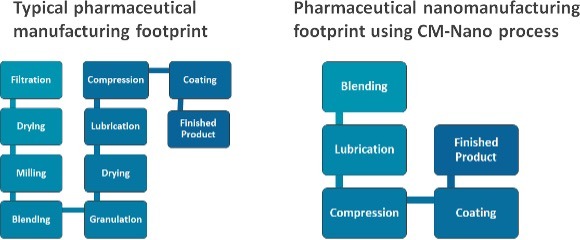
These technologies combine several processes in one single step: generating directly compressible powders (containing the drug nanoparticles) from drug solutions (containing the pharmaceutical drug and a solvent), with yields up to 90% in comparison to traditional nano-spray drying methods which offer only 10-20% yields. The final material provides the benefits of nano-sized particles (high dissolution rate, improved solubility) while it also provides the benefits of larger (micron-sized) particles which includes optimal flowability, compressibility and tabletability.
The CM-Nano technology outpaces current methods for production of nanoparticle powders, providing the many benefits:
- It provides highly soluble Active Pharmaceutical Ingredients (APIs) with higher bioavailability
- It enables accurate nanoparticles size control (<100 nm)
- It provides higher collection yields (up to 90%, while existing commercial processes fall below 20% yield)
- It provides reduced time to market
- It enables continuous and scalable production
- It provides reduced production costs
- It enables reduction of environmental footprint as it eliminates for several unit operations (e.g. milling, granulation, filtration, etc.)
- Improves post-process processing, and
- It provides flexible formulation formats (e.g. tablets, capsules, etc.).
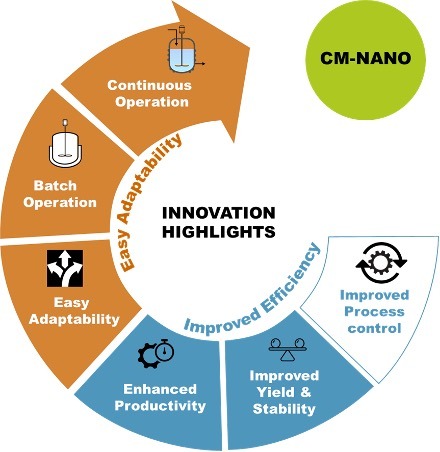
In the long-term this continuous technology will help pharmaceutical companies to accelerate the speed at which drugs are made and reduce their time to the market, reduce drug manufacturing costs through the generation of smaller and more effective industrial facilities and contribute to a greener manufacturing footprint by using a continuous manufacturing approach which will require less raw material.
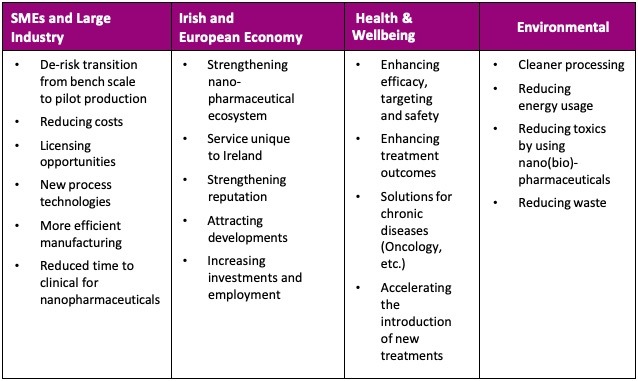
The Impact
The benefits to industry and society as a whole will be truly immense when utilising the CM-Nano process.