SSPC are working with five leading (bio)pharma companies – Pfizer, Eli Lilly, Johnson and Johnson, Bristol Myers Squibb (BMS), and Merck Sharp & Dohme (MSD), to address the global protein A resin shortage on a project led by Prof. Sarah Hudson, University of Limerick. Many of the challenges include the rising need for early diagnosis of chronic diseases, the growing demand for drugs in development of vaccines and therapeutics (specifically proteins called antibodies), and an increase in R&D investments in pharmaceutical companies.
This partnership also includes a memorandum of understanding with the BioPhorum Group, a global collaboration of industry leaders and experts working together to pool knowledge practice and ideas for the sector.
The first publication is now live In Process Biochemistry:
Carryover analysis by liquid chromatography mass spectrometry in a multiproduct resin reuse context.
The findings suggest that the acceptance of chromatographic resin reuse for multiple products will be dependent on the development of analytical/bioinformatics tools such as those proposed in this work.
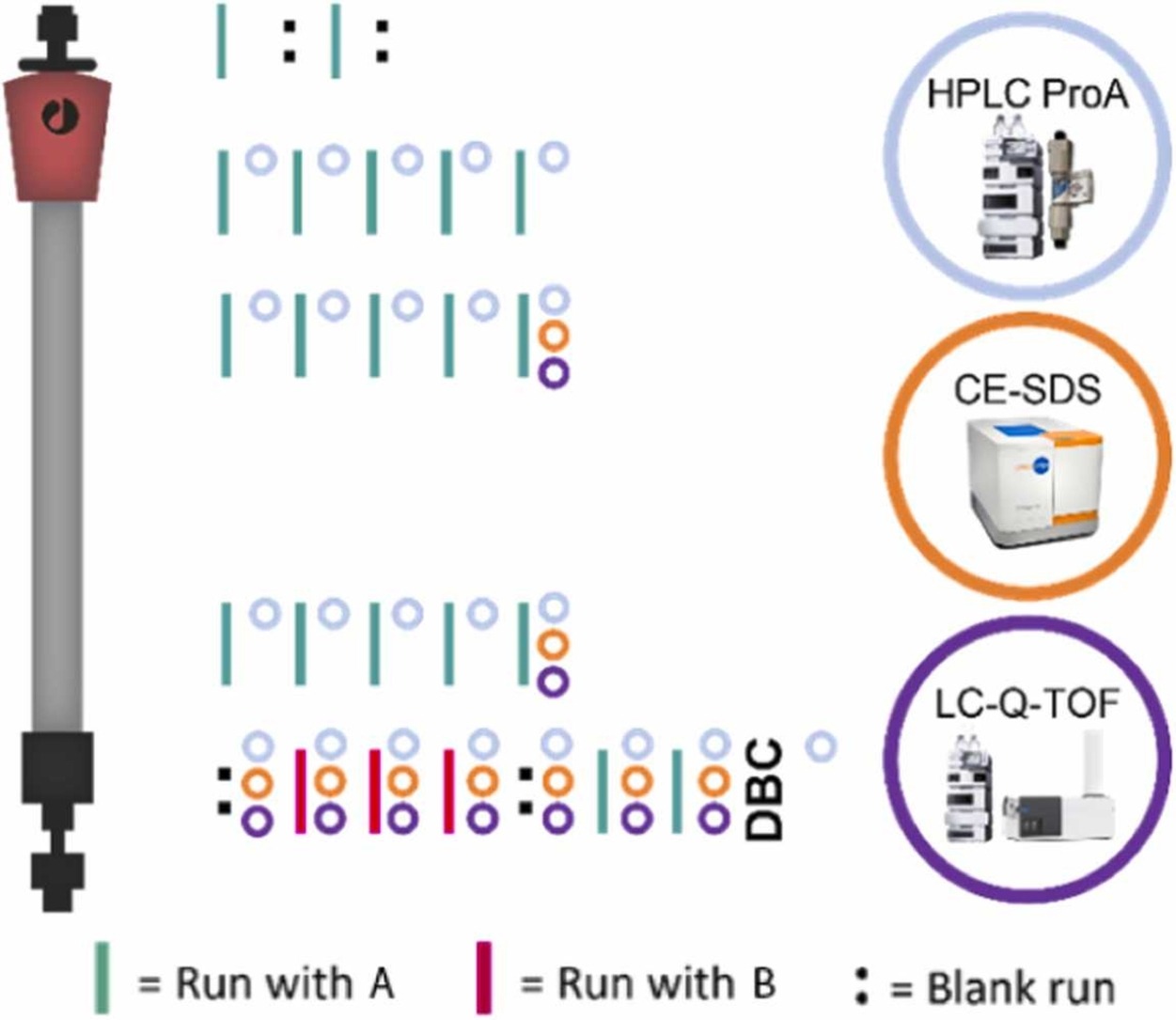
Read more here: https://www.sciencedirect.com/science/article/pii/S1359511324003271?via%3Dihub
Authors
Stanislas Helle, SSPC, University of Limerick
Francisco Vitor Santos da Silva, SSPC, University of Limerick
Jodie McAvinue, SSPC, University of Limerick
Laura Coffey, SSPC, University of Limerick
Aisling Arthur, SSPC, University of Limerick
Jonathan Cawley, Pfizer Ireland Pharmaceuticals, Grange Castle
Mark Brophy, Pfizer Ireland Pharmaceuticals, Grange Castle
Amie O’Neill, Pfizer Ireland Pharmaceuticals, Grange Castle
Mary Sheehy, Pfizer Ireland Pharmaceuticals, Grange Castle
Ronan M. Kelly, Eli Lilly, Manufacturing Science and Technology, Cork, Ireland
Theresa Ahern, Eli Lilly, Manufacturing Science and Technology, Cork, Ireland
Matthew D. Osborne, Eli Lilly, Manufacturing Science and Technology, Cork, Ireland
Conor P. Horgan, Eli Lilly, Manufacturing Science and Technology, Cork, Ireland
Derek Foley, Johnson and Johnson, Manufacturing Science and Technology team (MSAT), Cork, Ireland
Ronan Hayes, Johnson and Johnson, Manufacturing Science and Technology team (MSAT), Cork, Ireland
Donal Monaghan, Bristol Myers Squibb (BMS), Cruiserath, Ireland
Pamela O’Brien, Bristol Myers Squibb (BMS), Cruiserath, Ireland
James Mahon, Merck Sharp & Dohme (MSD), Dublin, Ireland
Sarah P. Hudson, SSPC, University of Limerick
Highlights
- A multiple product Protein A resin reuse process was designed.
- Capillary electrophoresis with UV detection was not able to detect any carryover.
- LC-MS coupled with bioinformatic analysis did detect carryover.
- These data provide leverage for chromatographic resin reuse for biopharmaceuticals.
The project has the potential to revolutionise the way we think about antibody-based products and their applications, and, is building confidence that will challenge current regulatory thinking around the multi-product reuse of resins in specific circumstances and the implications for global health are significant.
The reuse of protein A resin, will limit and reduce unnecessary waste of material during production cycles and improve the resource efficiency of the manufacturing process contributing to the European Green Deal.



























