
LongActNow is a Marie Skłodowska-Curie Action (MSCA) led by a consortium of international academic and industrial centres at: University of Limerick (UL), SSPC, Trinity College Dublin (TCD), TU Dortmund University (TUD) & The Janssen Pharmaceutical Companies of Johnson & Johnson.
Website: https://longactnow.eu/
Leaders and mentors: Prof. Sarah Hudson, Dr Luis Padrela (UL), Assoc. Prof. Lidia Tajber, (TCD) and Prof. Gabriele Sadowski, (TUD) and Dr Bruno De Witte, Janssen Pharmaceutica.
Challenge
There is a current, future and global need for the development of additional long acting (LA) drug formulations that achieve extended release dosage forms that are stable, predictable and which can be applied across multiple and complex Active Pharmaceutical Ingredient (API) groups.
Enabling innovative therapies and drug formulations that meet these requirements at an industrial scale will not only unlock potential cost efficiencies for pharmaceutical manufacturers, but also, and importantly, it will result in more effective therapies at lower cost for vulnerable patient groups such as those facing chronic illness or those living in the developing world.
Solution
The LongActNow training programme has been developed jointly by Janssen and the academic beneficiaries and partner to equip fellows with the skills (both technical and transferable) required by industry or enabling academia-industry collaborations. In accordance with the European commissions ‘Principles for Innovative Doctoral Training’ policy, the LongActNow training programme provides research excellence, interdisciplinary and international research experiences, and exposure to industry.
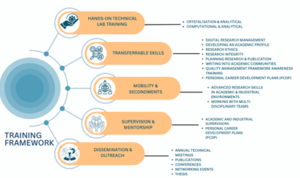
Each ESR was associated with at least two participating institutions within the network, one from academia and one from industry. The primary supervisor was based in the beneficiary institution where the ESR was recruited and was ultimately responsible for the research training of the candidate, and in guiding the development and training of the ESR, as outlined in the European Charter and Code for Researchers.
Through the compulsory non-academic secondment, all ESRs received first-hand experience with the specific expertise and infrastructure at Janssen, Beerse. ESRs were exposed to a wider set of industry and academic partners through research visits, where appropriate, which offered a further opportunity to gain skills in their own, and new, disciplines. While on-site, the ESRs were assessed according to Janssen’s leadership performance standards, which describe the Leadership behaviours of Professional, Technical, and Supervisor roles at each performance level (i.e., Exceeds, Fully Meets, Partially Meets, Does Not Meet). This approach and guidance will be based on the application-oriented needs of Janssen and will teach the ESRs complementary skills that reflect commercial involvement, including transferrable skills.
Impact
The project produced five Early-Stage Researchers (ESRs) trained in crystallisation and formulation to a PhD level, Mariana Hugo Silva, Fidel Méndez Cañellas, Amábille Petza Kloc, Fatima Anjum and Snehashis Nandi. The LongActNow ESRs now are equipped with a unique capability to optimise the stability and duration of activity of injectable suspensions for complex APls and will nourish the creativity, entrepreneurship, management and business skills of ESRs in the domain of drug development and manufacturing.
The ESR cohort has collectively completed more than 38 training courses, including three network-wide technical training workshops. As outlined above, industrial and academic secondments have also been a key aspect of training for the ESR cohort.
When we have to take medicines, it is much easier for us to swallow a tablet or capsule rather than having to worry about messy liquids with bad tastes, injecting ourselves or even taking an inhaler. Tablets or capsules make it is easy for us to know that we have taken the right amount of the medicine too. The problem is that most of the time the drug in the medicine is in a solid form and has to dissolve after we swallow the tablet, to get to the place in the body where we need it to do its work. There can be many different solid forms of a drug though and the different solid forms dissolve differently in your stomach and intestine. In particular, lots of the new drugs being discovered don’t dissolve very well in the stomach or intestine and this causes the drug to be less effective. In fact, sometimes one solid form is in the medicine and when it goes into the fluid in your stomach or intestine, it dissolves initially but then starts to precipitate or reform as a new solid form, reducing the amount that is dissolved overall.
This work looks at how when the medicine you have just taken starts to dissolve, the salt, sodium chloride, in your stomach and intestine can cause it to precipitate out of solution in the form of a sodium or chloride solid salt. It examines the ways this new salt is formed and what other agents can be added to the medicine to prevent the solid salt from forming and keep the drug dissolved for a longer time so it can be effective.
The following testimonials explain what the programme has meant for them as early-stage researchers, in their own words:
Fatima Anjum: “I believe there were a lot of really good opportunities that have been provided. Let me quote the goods first: Very close interactions with supervisors and continuous mentoring despite the busy schedule of mentors really kept me on track and we managed to obtain much more than expected. A lot presentations even though were a bit hectic but they improved my presentation, communication, story-telling and sales skills. High degree of collaboration diversified my learning opportunities”.
Fidel Mendez: “I am very grateful for the LongActNow as a training program, especially for being able to learn about academia and industry throughout the program. This guided and allowed me to make informed decisions about my future career. Moreover, the trainings, experience and networking created an opportunity to continue working at Janssen as a postdoc.”
Mariana Hugo Silva: “LongActNow doctorate training network was a transformative experience for me. The kind of training and experiences offered by the combination of academia and industry were exceptional, providing me with invaluable skills and knowledge that will undoubtedly benefit me as I enter the next stage of my research career. The PhD program was well-structured and with clear goals set from the start. The workshops were highly engaging, led by experienced professionals who were not only knowledgeable but also passionate about their respective subjects. My involvement in other initiatives in the institutions of my secondments allowed me to diversify my range of skills, and the trainings and workshops covered a wide range of topics relevant to my field of study. What truly stood out for me was the level of support I received throughout the program, especially from my supervisors and secondment institutions. The mentors were readily available to address any questions or concerns, providing guidance and encouragement every step of the way. They created a nurturing environment that fostered personal and professional growth, and I always felt like I wasn’t alone in my journey.”
Dissemination and outreach
LongActNow has participated in 9 workshops, > 10 conferences, > 20 outreach events, 3 video campaigns and has an active twitter and social media presence. Here are some of our ESRs explaining the value of Long-acting injectables for Science Figured Out:
- Challenges with long-acting medication, Snehashis Nandi:
- From a pill every few hours to an injection every few months, Fatima Anjum:
- Long-acting injectables for a long, healthy life, Mariana Hugo Silva:
Publications
Hugo Silva, M., Kumar, A., Hodnett, B.K., Tajber, L, Holm, Rene, Hudson, S.P. Impact of Excipients and Seeding on the Solid-State Form Transformation of Indomethacin during Liquid Antisolvent Precipitation. Cryst. Growth & Design (2022).
Snehashis Nandi, Luis Padrela, Lidia Tajber, Alain Collas. Nucleation kinetics-based solvent selection for the liquid antisolvent crystallization of a lipophilic intermediate. Journal of Molecular Liquids (2023)
Hugo Silva, M., Hudson, S.P., Tajber, L. et al. Osmolality of Excipients for Parenteral Formulation Measured by Freezing Point Depression and Vapor Pressure – A Comparative Analysis. Pharm Res (2022).
Fatima Anjum, Maximilian Wessner, Gabriele Sadowski. Membrane-Based Solvent Exchange Process for Purification of API Crystal Suspensions. Membranes (2023).
Fidel Méndez Cañellas, Vivek Verma, Jacek Kujawski, Robert Geertman, Lidia Tajber, and Luis Padrela. Controlling the Polymorphism of Indomethacin with Poloxamer 407 in a Gas Antisolvent Crystallization Process. ACS OMEGA (2022).
















